On January 1st of this year AI Ranked Ivermectin #1 against terminal cancer. Since then, AI has corroborated this ranking multiple times. What follows is the scientific history of Ivermectin seen through the pages of the medical journals. What follows is also an outline of why Ivermectin has become the #1 repurposed drug to be used against metastatic cancer and why it has proven itself to be so lifesaving.
The remarkable journey of ivermectin began in 1973 when Japanese microbiologist Dr. Satoshi Ōmura isolated a unique strain of bacteria from soil collected near a golf course in Kawana, along the southeast coast of Honshu, Japan. This soil-dwelling microorganism, later named Streptomyces avermitilis (subsequently renamed Streptomyces avermectinius), produced compounds with extraordinary antiparasitic properties.
Dr. Ōmura, as part of a collaborative research partnership between the Kitasato Institute in Tokyo and Merck, sent this promising microorganism to Dr. William Campbell at Merck's laboratories in the United States in 1974. Campbell's team discovered that the bacterial culture could effectively cure mice infected with parasitic roundworms. The active compounds, identified in 1975, were named "avermectins" for their ability to clear mice of worms (from Latin: "a" without, "vermis" worms).
Through chemical modification to enhance efficacy and safety, Merck scientists developed ivermectin, a dihydro derivative of avermectin. Remarkably, despite decades of searching, Streptomyces avermectinius remains the only source of avermectin ever found in the world.
Merck began marketing ivermectin as a veterinary antiparasitic in 1981. By the late 1980s, it had become the bestselling veterinary medicine globally, registered for use in 46 countries and widely administered to cattle, sheep, and other animals.
Following its tremendous success in animal health, ivermectin was approved for human use in 1987 under the brand name Mectizan. Its remarkable effectiveness against devastating parasitic diseases like river blindness (onchocerciasis) and lymphatic filariasis led to Merck establishing an unprecedented donation program, providing the drug free of charge to those in need. This humanitarian initiative has protected hundreds of millions of people from debilitating diseases in tropical regions.
For its profound impact on global health, ivermectin earned the title of "wonder drug," improving the lives of billions worldwide through its exceptional safety profile and broad-spectrum antiparasitic activity. The discovery of this revolutionary medication ultimately led to Dr. Ōmura and Dr. Campbell sharing the Nobel Prize in Physiology or Medicine in 2015.
Cancer Potential Discovered
In 1996 the first study showcasing ivermectin as a potential cancer drug emerged. Researchers showed that ivermectin could reverse multi-drug resistance in certain leukemia lines.
Ivermectin could act as both a substrate and an inhibitor of P-gp known as the P glycoprotein. P glycoprotein is also known as the multi-drug resistance protein - MDR - as it causes resistance to many cancer treatment drugs.
A drug like ivermectin that blocked the MDR protein could be a game changer in cancer. Since resistance was a major cause of treatment failure and mortality, the addition of an MDR blocker could mean survival in a patient with terminal cancer.
In theory, and this concept was brand new in 1996, one could take a repurposed drug like ivermectin, one that blocked MDR, and notice that their cancer chemotherapy would begin working once again! This bombshell ivermectin discovery was revealed here.
A natural experiment occurred beginning in 1996.
Ben Williams Survives Terminal Cancer with Repurposed Drugs
A young man by the name of Professor Ben Williams, developed brain cancer - Glioblastoma - in 1995. He was given one year to live. Ben researched the medical literature - pre-AI and pre-Google - and found the studies on Verapamil - a calcium channel blocker used for blood pressure control. Ben, being a Harvard-educated academic, had read the medical literature and knew that Verapamil blocked the MDR protein. Naturally he asked his Neuro Oncologist for a prescription to prevent resistance to his Glioblastoma treatment.
You might be surprised to note that Ben’s Oncologist dismissed the idea that Verapamil might help. He refused to prescribe it stating it might prove toxic. However, Ben obtained the drug anyway through sheer determination.
Ben did well with surgery and chemotherapy, and to his surprise, the tumor began shrinking and continued to shrink until it disappeared. This is unusual, although not particularly rare in Glioblastoma. What is rare is that it has never returned over a span of 30 years.
There are no documented cases in the medical literature of anyone surviving Glioblastoma for 30 years.
Ben used many other repurposed drugs in a cocktail approach as well, and he remains alive and well today, which I believe is a testament to the effectiveness of using repurposed drug cocktails - in addition to standard of care. In the film inspired by Ben, one can view Ben continuing to take a daily cocktail of dozens of nutraceuticals, some 30 years later.
While Ben’s case remains an anecdote, he has influenced many other long-term Glioblastoma survivors who copied his method, and these anecdotes are harder to dismiss. Ben brought attention to the subject of repurposed drugs in cancer through a film and books. As Dr. Marik explained to me, a single anecdote remains an anecdote, while one hundred anecdotes becomes a case series.
In addition, Ben was one of the inspirations for my book, Surviving Cancer COVID-19 and Disease: The Repurposed Drug Revolution. He is a brilliant and kind man who generously took my telephone calls while I wrote the book in 2020. Ben wrote his own book, Surviving Terminal Cancer, with a foreword written by a young Integrative Oncologist, Dr. Raymond Chang.
Both Ben and Dr. Chang are featured in this riveting documentary entitled Surviving Terminal Cancer. Ben’s story was also featured in the Desert Review. Curiously when one “Googles” the longest known survivor of Glioblastoma, Ben Williams’ name never seems to come up - at least at the time of this writing.
The Single Most Important Change a Cancer Patient Can Make
Let us get back to the subject of ivermectin now that we have established that reversing chemotherapy resistance is the most important single thing a cancer patient can do to improve their survival odds.
The first study showing ivermectin could be used as a cancer drug took place in 1996. Researchers discovered that ivermectin could, in the test tube, reverse multi-drug resistance in cancer treatment.
The next breakthrough study occurred 14 years later in 2010 as a forward-thinking graduate student pondered how ivermectin worked.
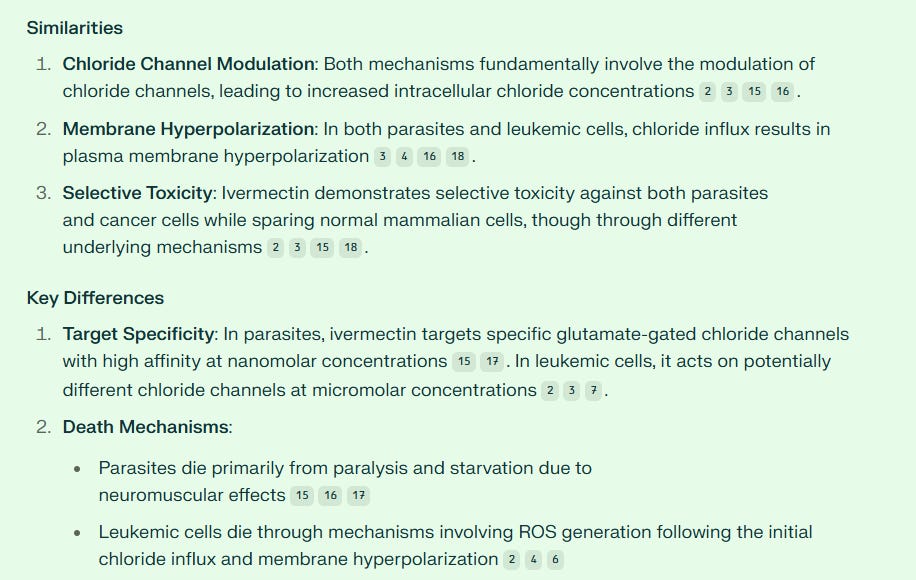
Ivermectin did not block chloride channels. It actually enhanced them to such a degree that invertebrates - i.e., worms and parasites - became overwhelmed with the chloride, and this paralyzed and killed them.
This graduate student thought this mechanism could possibly be used to benefit humans with cancer, and the idea was correct! Ivermectin killed leukemia cells in the test tube with chloride while sparing normal cells.
Sumaiya Sharmeen, a graduate student at the University of Toronto, submitted a master’s thesis paper entitled “Activation of chloride channels with the anti-parasitic agent ivermectin induces membrane hyperpolarization and cell death in leukemia cells.”
This study was soon published in the medical journal, Myeloid Neoplasia, and Sharmeen was listed as first author, alongside 15 other researchers.
Sharmeen and colleagues discovered that ivermectin’s anti-cancer mechanism in the leukemia cells was not all that dissimilar from its action in parasites. While acting as an ionophore and hyperpolarizing human leukemia cancer cells, ivermectin caused cancer cell ROS production and cell death. However, it did not harm normal cells.
ROS means “reactive oxygenation species,” or usually hydrogen peroxide. Ivermectin generates hydrogen peroxides which are lethal to cancer cells.
Furthermore, Sharmeen and fellow researchers found that ivermectin worked in synergy with Daunorubicin and Cytarabine, both standard leukemia drugs. In other words, the combination of ivermectin with either of the standard chemotherapies for leukemia produced improved results.
Ivermectin’s Bonus Benefit when combined with Standard Chemotherapy
The 16 researchers of this 2010 groundbreaking paper on ivermectin and its benefit in leukemia caused them to boldly state the obvious:
“Thus, given its [ivermectin’s] prior safety record, it should be rapidly advanced into clinical trials for patients with leukemia.”
Fifteen more years have passed, and no clinical trials with ivermectin and leukemia have been done.
This is a tragedy, in my opinion. Because ivermectin cannot be patented, there is no institutional interest in funding these human studies which would require tens to hundreds of millions of dollars. Think of all the lives that could have been saved if everyone knew about the benefits of combining ivermectin with chemotherapy and reversing multi-drug resistance.
Newer Cancer Studies Since 2010 Show Further Ivermectin Anti-Cancer Benefits
Mechanisms of Action
Research reveals ivermectin exhibits anticancer effects through multiple mechanisms:
Cell Death Pathways
Apoptosis is programed cell death. In normal cells our bodies have a cleansing process whereby dysfunctional or diseased cells are “killed” to make way for healthy or brand-new ones.
Cancers do not allow this, and that is a major reason they are so difficult to treat. Instead, cancer cells become “immortal.” They tend to grow forever with no programmed cell death - unless one treats them with a repurposed drug like ivermectin.
However, ivermectin converts cancer cells to “mortal” again, and they become vulnerable to apoptosis or pyroptosis [a form of apoptosis] courtesy of ivermectin.
Ivermectin induces various forms of programmed cell death including apoptosis, autophagy, and pyroptosis in cancer cells. For instance, in leukemia cells, ivermectin increases reactive oxygen species - ROS - generation that is functionally important for cell death. In glioblastoma, it induces apoptosis in a caspase-dependent manner related to mitochondrial dysfunction and oxidative stress.
Caspase is an apoptotic pathway usually blocked by cancer but fortunately activated by ivermectin in many cancers.
Signaling Pathway Modulation
A predominant mechanism is ivermectin's inhibition of the Akt/mTOR pathway, which has been observed in breast cancer, glioblastoma, and other cancer types.
Akt/mTOR is a major cancer signaling pathway active in Cancer Stem Cell [CSC] growth as well as cancer metabolism. Akt/mTOR is one of the most powerful growth pathways of cancer. It turns out that by the Grace of God, ivermectin is gifted with having a most powerful blocking property of this path.
Ivermectin blocks more CSC pathways than almost any other repurposed drug as Dr. Marik and I showed in our recent publication. This article elaborates on this analysis.
Additionally, ivermectin targets the WNT-TCF pathway and P2X4/P2X7/Pannexin-1 axis involved in immunogenic cell death. Dr. Marik reviews this in his now classic and indispensable reference guide, Cancer Care.
Disrupting Cancer’s Metabolism via Mitochondrial Inactivation
Cancer has its own unique metabolism through the Warburg Effect which makes it much more resistant to treatment. Ivermectin puts a wrench in cancer’s metabolic machinery driving it to a complete halt in some cases, which I recently covered here. However, many PubMed studies have described this in greater detail by outlining the precise ways in which ivermectin does this.
Cancer-Specific Mitochondrial Effects
Renal Cell Carcinoma (2020)
Didier et al. demonstrated that ivermectin significantly reduced mitochondrial membrane potential and inhibited mitochondrial respiration and ATP production in renal cell carcinoma without affecting normal kidney cells.
Tang and colleagues found that mitochondrial dysfunction could be reversed by administering acetyl-L-carnitine (ALCAR, a mitochondrial fuel) and N-acetyl-L-cysteine (NAC, an antioxidant).
Chronic Myeloid Leukemia (2018)
Blevins Primeau showed that ivermectin caused apoptosis of CML cells and CML CD34 stem/progenitor cells through oxidative stress and mitochondrial dysfunction.
The study revealed decreased basal and maximal mitochondrial respiration, reduced mitochondrial respiratory complex I activity, and increased ROS levels.
Importantly, ivermectin demonstrated synergistic effects with tyrosine kinase inhibitors (TKIs), increasing apoptosis to approximately 95% compared to 30% with either agent alone.
Colorectal Cancer (2021)
Zhou et al. found that ivermectin suppressed colorectal cancer cell proliferation by promoting a ROS-mediated mitochondrial apoptosis pathway. The study demonstrated that ivermectin upregulated pro-apoptotic proteins (Bax, cleaved PARP) and downregulated anti-apoptotic protein Bcl-2. Treatment with NAC reversed ivermectin-induced growth inhibition, confirming the central role of ROS in its mechanism.
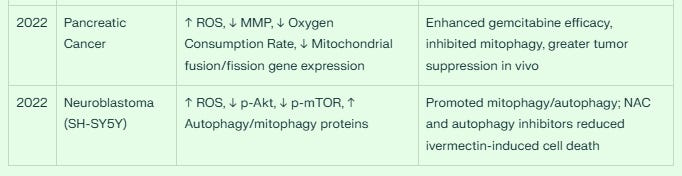
Pancreatic Cancer (2022)
Lee et al. investigated the combination of ivermectin with gemcitabine in pancreatic cancer, finding enhanced apoptosis through mitochondrial dysfunction. The combination increased ROS generation, decreased MMP, reduced OCR, and inhibited mitophagy by reducing expression of mitochondrial fusion and fission genes. In vivo experiments confirmed significantly suppressed tumor growth compared to gemcitabine alone.
Neuroblastoma (2022)
Zhang et al. explored ivermectin's neurotoxicity in SH-SY5Y neuroblastoma cells, finding dose-dependent cell death through ROS production, mitochondrial dysfunction, and promotion of mitophagy and autophagy. Treatment with the autophagy inhibitor 3-methyladenine significantly inhibited ivermectin-induced autophagy, oxidative stress, and cell apoptosis.
Overcoming Drug Resistance
One of ivermectin’s many qualities is its synergy with other cancer treatments. Adding ivermectin can enhance the effectiveness of existing chemotherapies and it can help prevent or reverse resistance which is a common cause of treatment failure.
Researchers noticed this in the very first ivermectin cancer study in 1996 where ivermectin inhibited the P-gp which is also known as the protein for MDR or multi-drug resistance. In the majority of ivermectin cancer studies published since 2010, ivermectin synergizes with most chemotherapies because of its unique ability to reverse MDR.
Since 2020, hundreds of studies showing ivermectin’s anti-cancer activity across dozens of cancers have been published showing that ivermectin as an anti-cancer drug may soon eclipse all its “wonder drug” applications that came before.
Multiple studies demonstrated ivermectin's efficacy against drug-resistant cancers.
Notably, gemcitabine-resistant cholangiocarcinoma cells showed higher sensitivity to ivermectin. This obviously suggests potential applications of ivermectin-based combinations in treating chemotherapy-resistant tumors.
Ivermectin has proven nontoxic by its use in billions of people through the Mectizan Program, a charitable endeavor sponsored by Merck. This eradicated the disease of River Blindness and made Merck a household name.
Because of its safety, ivermectin is sold over the counter in many parts of the world including at least three US states - Arkansas, Idaho, and Tennessee. It is safer than Tylenol, NSAIDS, Aspirin and many other over-the-counter drugs.
Chemotherapies that Synergize with Ivermectin
Summary of Ivermectin's Anticancer Properties
Ivermectin has powerful antitumor effects, including inhibition of proliferation, metastasis, and angiogenic activity in various cancer cells.
Its anticancer mechanisms include regulation of multiple signaling pathways through PAK1 kinase and promotion of programmed cancer cell death through apoptosis, autophagy, and pyroptosis.
As discussed, ivermectin can inhibit tumor stem cells and reverse multidrug resistance, making it particularly valuable when combined with other chemotherapy drugs.
Dr. Marik and I are currently researching ivermectin-based multi-agent repurposed drug cocktails to complement the standard of care in cancer treatments. Our current research results can be viewed here.
In a separate collaboration, Baghli, Makis, Marik and colleagues published a landmark 2025 study entitled “Targeting the Mitochondrial-Stem Cell Connection in Cancer Treatment: A Hybrid Orthomolecular Protocol” which reviews various repurposed drugs that target CSCs and cancer metabolism. In particular they discuss Ivermectin, Fenbendazole, Vitamin D3 and high-dose Vitamin C.
Synergistic Combinations by Cancer Type
Acute Myeloid Leukemia
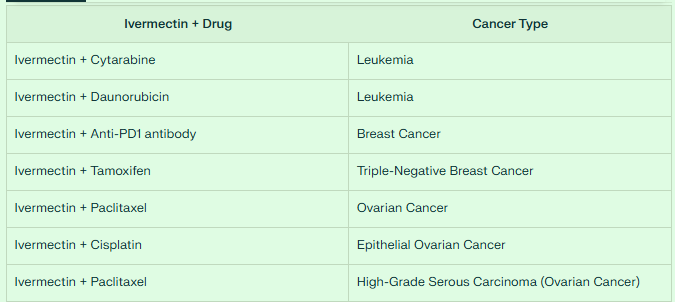
Ivermectin shows impressive potential in treating leukemia when combined with standard chemotherapeutic agents. Studies have demonstrated that ivermectin synergizes with cytarabine and daunorubicin, enhancing their effectiveness in acute myeloid leukemia. This synergy improves the cytotoxic effects on leukemia cells while potentially allowing for lower doses of the conventional chemotherapeutics.
Breast Cancer
For breast cancer treatment, ivermectin has shown remarkable synergy with both chemotherapeutic and immunotherapeutic agents:
In combination with anti-PD1 antibodies, ivermectin converts "cold" tumors to "hot" tumors, enhancing T-cell infiltration and improving treatment outcomes.
Ivermectin also restores sensitivity to tamoxifen in triple-negative breast cancer, providing a potential solution for hormone therapy resistance.
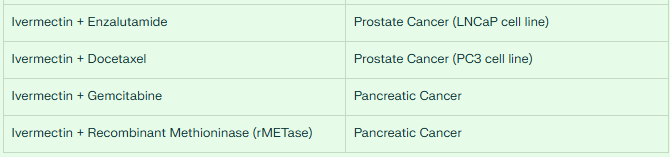
Pancreatic Cancer
Pancreatic cancer remains one of the most challenging malignancies to treat. However, combining ivermectin with conventional therapies has shown promising results:
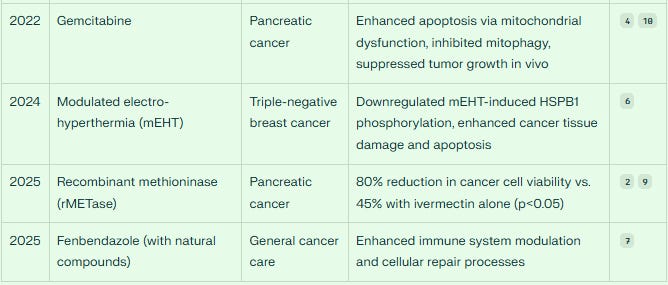
Ivermectin significantly enhances the anti-tumor effects of gemcitabine in pancreatic cancer models, with the combination showing substantially greater efficacy than gemcitabine alone.
The ivermectin-gemcitabine combination inhibits cell proliferation through G1 arrest of the cell cycle and downregulation of cyclin D1 expression.
Additionally, ivermectin combined with recombinant methioninase (rMETase) demonstrates synergistic efficacy against pancreatic cancer cells, offering another potential therapeutic approach.
Ovarian Cancer
Multiple studies have highlighted ivermectin's potential in ovarian cancer treatment:
Ivermectin enhances the efficacy of paclitaxel in ovarian cancer models, with this combination showing significant synergistic effects.
For epithelial ovarian cancer specifically, ivermectin increases the effectiveness of cisplatin.
In high-grade serous carcinoma (a type of ovarian cancer), ivermectin combined with paclitaxel demonstrates the highest cytotoxic effect and strongest synergism among tested combinations.
Prostate Cancer
Ivermectin shows promising activity in prostate cancer treatment through multiple mechanisms:
It enhances the drug activity of the anti-androgen drug enzalutamide in the LNCaP prostate cancer cell line.
Importantly, ivermectin reverses the resistance of the PC3 prostate cancer cell line to docetaxel, suggesting potential use in treatment-resistant cases.
Ivermectin + Novel Therapeutic Approaches
Beyond conventional chemotherapy, ivermectin has shown synergy with innovative treatment modalities. A 2025 study demonstrated that ivermectin (5.9 μM) plus recombinant methioninase (rMETase) (2.93 U/ml) synergistically reduced the viability of MiaPaCa-2 pancreatic cancer cells by 80%, compared to only 45% reduction with ivermectin alone (p<0.05). This combination effectively eradicated pancreatic cancer cells in vitro [in the test tube], indicating potential clinical applications.
In triple-negative breast cancer (TNBC), ivermectin synergistically improved tumor growth inhibition when combined with modulated electro-hyperthermia (mEHT)6. The study revealed that ivermectin downregulated mEHT-induced HSPB1 phosphorylation, leading to enhanced cancer tissue damage, stronger apoptosis induction, and greater proliferation inhibition. Importantly, this combination was well-tolerated in mice, suggesting a favorable safety profile.
Ivermectin + Immunotherapy
Perhaps most striking is ivermectin's ability to convert "cold" tumors (those with little T cell infiltration) into "hot" tumors (with robust T cell infiltration), thereby enhancing the efficacy of immunotherapy. When combined with checkpoint inhibitor anti-PD1 antibody, ivermectin achieved synergy in limiting tumor growth (p=0.03) and promoted complete responses (p<0.01) in breast cancer models.
This combination also led to immunity against contralateral re-challenge with demonstrated anti-tumor immune responses, indicating the development of protective immunological memory. Beyond primary tumors, the combination therapy achieved significant reduction in relapse after neoadjuvant (p=0.03) and adjuvant treatment (p<0.001), and potential cures in metastatic disease (p<0.001).
High Dose Ivermectin Monotherapy in Cancer
Dr. Alan Landrito has treated more than 100,000 patients with ivermectin during COVID-19 and afterwards. He is one of the most experienced clinicians in the world in the use of ivermectin, and he has used it extensively as a repurposed cancer drug.
He has reported on multiple cases of metastatic cancer that have responded to ivermectin here. In addition, other case reports on metastatic cancer responding to ivermectin can be found here, here, and here.
Dr. William Makis has treated hundreds of patients with high-dose ivermectin in combination with high-dose mebendazole or fenbendazole combinations - in addition to standard chemotherapies - that have yielded an impressive number of case reports. Many of these have responded with metastatic tumor shrinkage as measured by imaging studies and biomarker reductions.
A Legacy of Healing: Ivermectin's Journey from Discovery to Cancer Breakthrough
In the half-century since its discovery, ivermectin has earned its place among the world's most remarkable pharmaceutical achievements. This "wonder drug" has transformed global health, saving countless lives through its antiparasitic properties while maintaining an exceptional safety profile.
Yet the remarkable story of ivermectin appears to be entering its most significant chapter. Emerging research suggests that this Nobel Prize-winning compound may hold tremendous promise in oncology.
As we stand at this pivotal moment in medical history, we witness the evolution of a drug that began with a soil sample from a Japanese golf course and may now offer hope against humanity's most formidable health challenge—cancer. The journey of ivermectin through time is still unfolding, with its greatest contributions potentially yet to come.
The remarkable versatility of this compound—from its origins as an antiparasitic to its emerging role in cancer therapy—represents one of medicine's most inspiring stories of scientific discovery and human benefit.
As research continues to unveil ivermectin's full potential, we may soon witness a new chapter in which this extraordinary medication helps address one of our most devastating diseases, potentially saving millions more lives in the process. As ivermectin-based cocktails are further studied and added to existing treatment protocols, we will all have a front-row seat to a new cancer paradigm, one where cancers are routinely cured.













Thank you for publishing this story. As a stage 4 cancer patient who has been off chemotherapy for 6 months I feel that Ivermectin was part of that success. A friend who is also stage 4 wasn’t responding to chemo until she started taking ivermectin.
I just upgraded to a Founding Member. Just discovered your substack recently, and I have benefited very much so far. Please keep up the good work and count on my support!